
Compared to +1 or -1 ions, +2 or -2 ions will release more lattice energy. Consequently, ions with weaker charges cause their compounds’ lattice energies to fall.Īs a result of their stronger attraction to one another, lattice energy rises as ion charge increases. Accordingly, ions with higher charge intensities will result in ionic compounds with higher lattice energies. The lattice energy increases as the ion charge variable is increased. As a result, they are less attracted, resulting in less lattice energy being produced. It is because when ions become larger, their nuclei become further apart. It reduces as halide size grows down the group.

The graph of lithium halide’s lattice energy is shown below. As a result, the lattice energy decreases as the ion radius grows. Larger lattice energies are produced in the ionic compounds of smaller ions. The increased distance between large ions generally results in ionic compounds with decreased lattice energies. Lattice energy diminishes as the distance variable is increased. Additionally, the size or radius of the ions, as well as the charge of the ions, affect the lattice energy. The ionic bond tenacity increases with an increase in the lattice energy. According to the second definition, creating an ionic compound requires exothermic lattice energy, holding a negative value. Because this process uses energy, it is considered endothermic, which holds a positive value.Įxothermic – When a process produces heat, it is exothermic. As per the initial definition, the structure of the lattice energy value changes with the breakdown of an ionic compound.
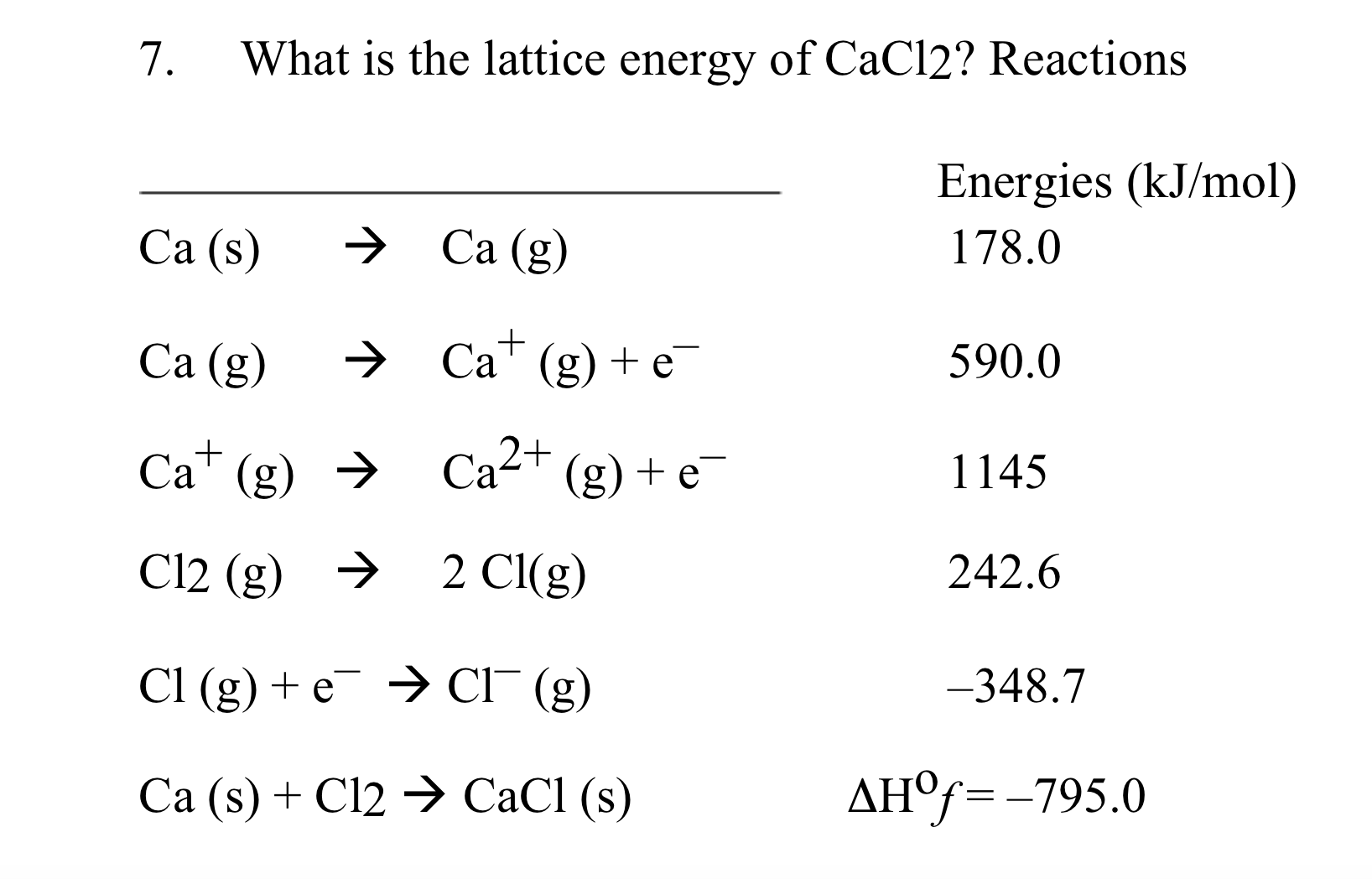
Exothermic or Endothermic?Įndothermic – We can see lattice energies as either exothermic or endothermic processes depending on the terminology we use. The lattice energy of a particular ionic molecule might either be a positive or negative value, based on the definition we use. It can also refer to the energy that enables one mole of a solid ionic compound to dissociate into its component gaseous ions. While some sources describe lattice energy differently, i.e., the amount of energy released during the exothermic formation of an ionic solid from its gaseous ionic components, this definition requires that lattice energy always has a negative value.Īs a result, it can be defined as the energy released when gaseous ions react to form one mole of a solid ionic compound. As a result, this amount has a positive value at all times. Therefore, we may write.Īlternatively, it can be viewed as the energy needed to endothermically break one mole of an ion crystal to gaseous ions in a space (vacuum). In other words, it is the energy generated when an anion and a cation unite to produce one mole of an ionic compound. The energy released during this procedure is called lattice energy. After creating ions, they come together to form an ionic compound. Ionic compounds are more persistent because of the electrostatic force present between the two opposing ions. The SI unit of lattice energy in kilojoules per mole (kJ/mol). However, it can be easily calculated using the Born-Haber cycle. Ionic solid lattice energy cannot be determined directly. It sheds light on several ionic solids’ characteristics, such as their volatility, solubility, and hardness.

Lattice energy evaluates the intensity of the ionic bonds present in an ionic compound.


 0 kommentar(er)
0 kommentar(er)
